Hemoglobin & Myoglobin: 4. Dissociation Curves - Biochemistry

Dissociation Curves We can compare compare the binding properties of both myoglobin and hemoglobin by drawing their dissociation curves. These curves measure their relative affinities for oxygen. • We draw a graph and label the x-axis oxygen partia
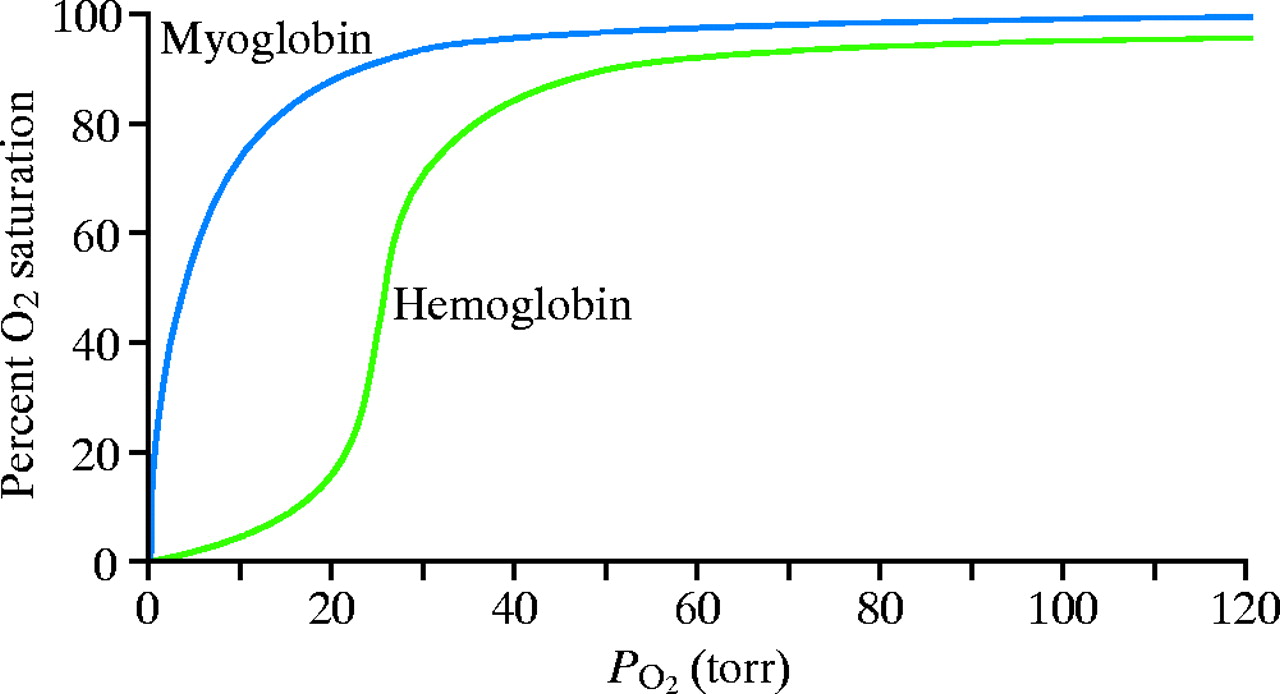
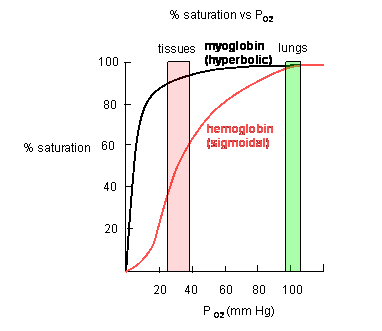
Dissociation Curves We can compare compare the binding properties of both myoglobin and hemoglobin by drawing their dissociation curves. These curves measure their relative affinities for oxygen. • We draw a graph and label the x-axis oxygen partial pressure (torr). Number it 0 to 120. – 30 torr is ~ the partial pressure of oxygen in the body's tissues. – 100 torr is ~ the partial pressure in the lungs. • The y-axis % oxygen saturation: it's numbered 0 to 100. We will use it to compare the oxygen binding patterns of both hemoglobin and myoglobin. oxygen binding curve for hemoglobin and myoglobin Hemoglobin • We draw a sigmoidal curve that plateaus just below 100% saturation. • Cooperative binding produces this sigmoidal shape. – As one oxygen molecule binds, hemoglobin's affinity for additional oxygen increases, and its percent saturation rapidly increases. • We show that hemoglobin reaches half saturation in the peripheral tissues – it responds to oxygen availability and releases it when partial pressure is low. Myoglobin • We draw a hyperbolic curve to the left of the hemoglobin curve, a much simpler binding pattern that corresponds to myoglobin's single heme group. – Myoglobin has a high affinity for oxygen, and does not release it until the partial pressure is very low. – These binding properties correspond to myoglobin's role in oxygen storage. – We label the early portion of the curve "Exercising muscle" and the plateau "Muscle at rest." Myoglobin vs. Hemoglobin • Myoglobin releases oxygen in response to the muscle's immediate needs. • Hemoglobin's cooperative binding allows it to respond to changes in oxygen availability. Fetal hemoglobin dissociation curve • As a clinical correlation, we show that the fetal hemoglobin dissociation curve is to the left of the adult hemoglobin curve. • This is because it has a greater affinity for oxygen to facilitate oxygen transfer from the maternal hemoglobin to the fetus; fetal oxygen supplies come from the mother.

HEMOGLOBIN Biochemistry (BMS 233) L.Noha Soliman. - ppt download
Why might an oxygen dissociation curve be sigmoidal?
What are the similarities between myoglobin and haemoglobin? - Quora

biochem unit 2 Flashcards

Oxygen–hemoglobin dissociation curve - Wikipedia

physiology - Cooperativity of haemoglobin and oxygen dissociation
ADD YOUR PAGE TITLE

PDF] Myoglobin oxygen dissociation by multiwavelength spectroscopy

Hemoglobin - Wikipedia
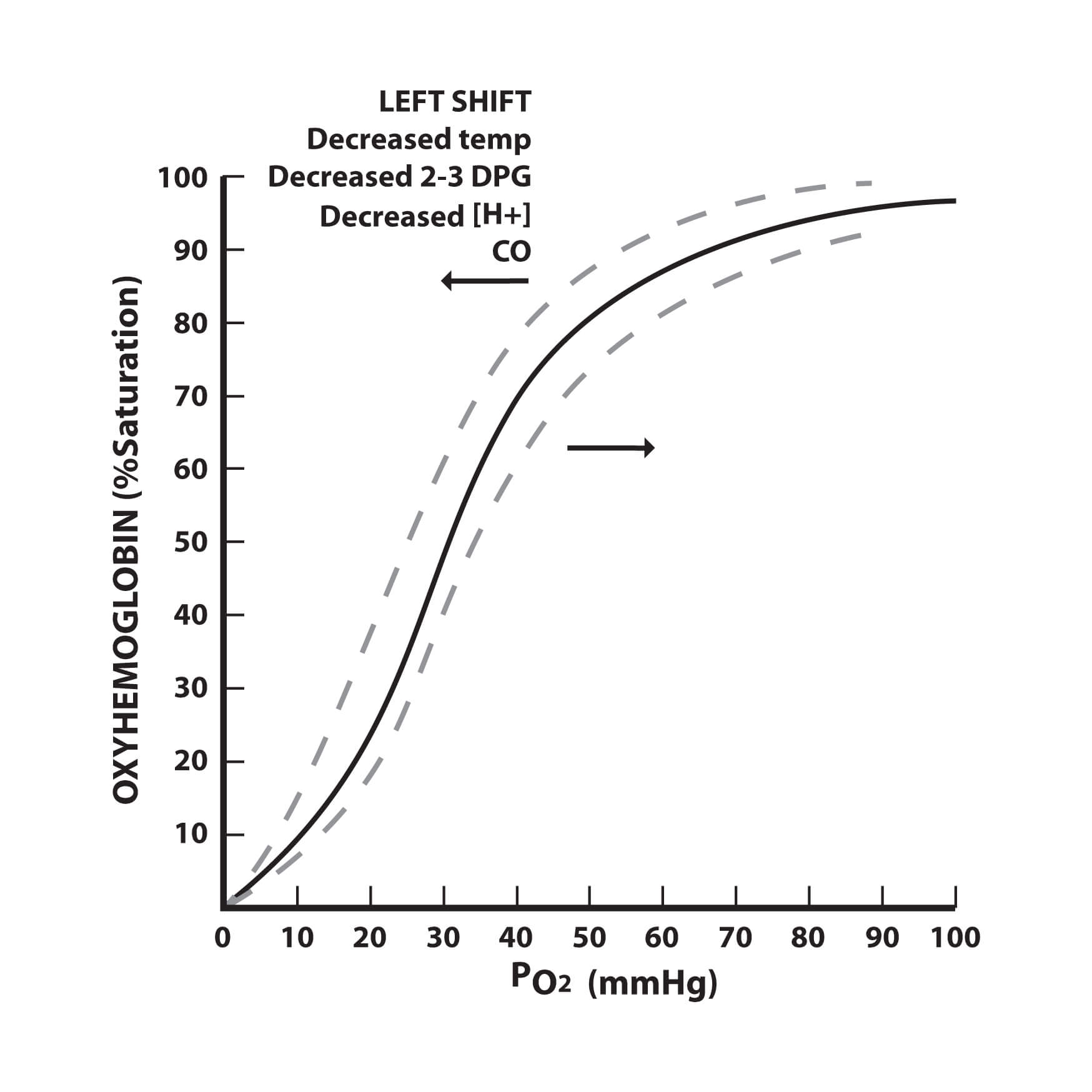
Understanding the Oxygen Dissociation Curve - Medical Exam Prep

Biochemistry I Chapter 7 Problems Flashcards

Myoglobin - an overview
Studies on the Oxidation-Reduction Potentials of Heme Proteins. IV

BIOCHEM EXAM 2 Flashcards




:max_bytes(150000):strip_icc()/Smoky-Sweet-Potatoes-With-Chorizo-Butter-FT-RECIPE1121-155da84b9f5349cbada35159ee8addef.jpg)