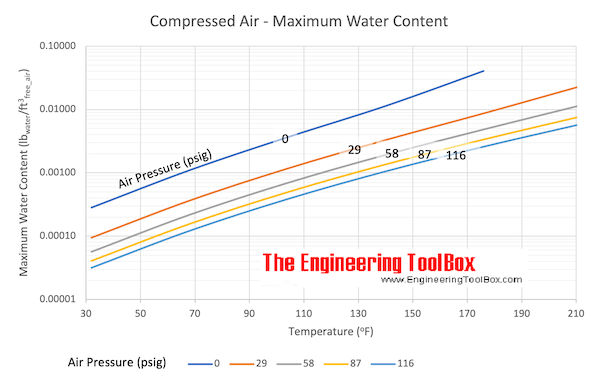
Why do pressure and temperature increase during the compression of

The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Learn more about this in this article.

Non-ideal behavior of gases (article)

Air at 100 KPa and 280 K is compressed steadily to 600 KPa and 400

thermodynamics - Water pressure vs temperature - Physics Stack
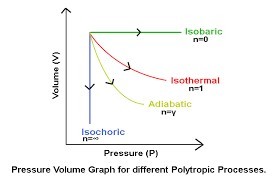
Adiabatic process PV^y = Constant, y = Cp/Cv = 3/2: A short

Boyle's law states that when a sample of gas is compressed at a
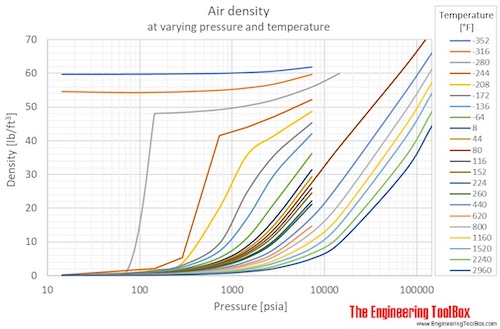
Air - Density vs. Pressure and Temperatures

Thermodynamic processes
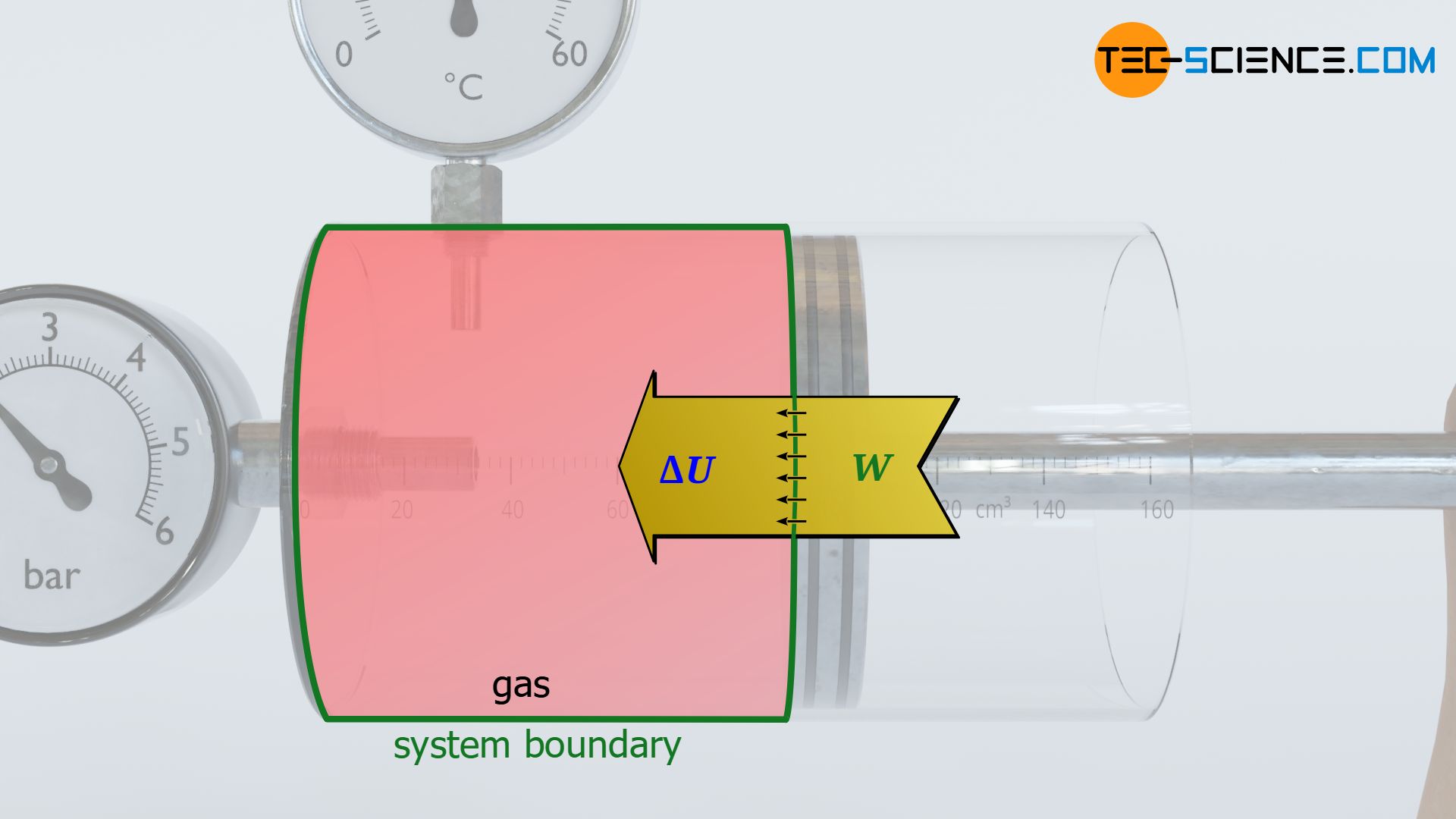
What is meant by dissipation of energy? - tec-science
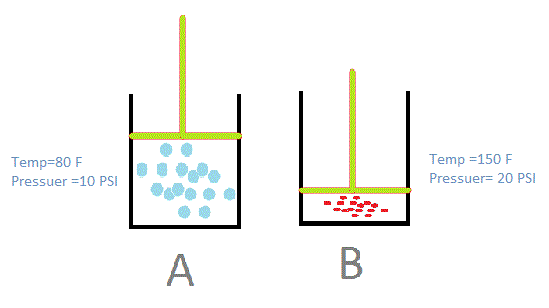
Thermodynamics Questions - #2 Page 433

Boyle's law, Definition, Equation, & Facts

adiabatic-reversible-expansion-and-compression-summary - LearnChemE

Solved Problem 1: Isothermal and adiabatic processes A. In






.jpg)